What Is Chelation?
Think of the peel of an orange. The peel protects the fruit from dirt, bugs, or drying out until you are ready to consume it. This is similar to how a chelate works. A chelate occurs when two chelating agents attach to an ion. Like the orange peel, the chelate protects the nutrient from combining with other elements or losing nutrient value for absorption.
Chelates provide many benefits including increased nutrient mobility, nutrient precipitation prevention, the avoidance of nutrient leaching, and more. We will explore these benefits along with how chelates help plant and animal nutrition but first we need to understand the science of chelation.
The Science of Chelation
Below is a typical structure shown for a chelate of glycine. Glycine is an amino acid we’ve developed a full animal feed line around due to its positive growth effects.
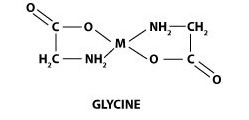
Note the M in the middle of the structure. The M represents a metal ion. Attached to the metal are two glycinates. Glycinates are natural chelating agents, that combine to the metal enabling the chelation process.
Chelating Agents
Usually an organic compound, chelating agents are organic molecules that can trap or encapsulate certain metal ions like Ca, Mg, Fe, Co, Cu, Zn, and Mn. These organic molecules can form several bonds with a single metal ion. Natural chelating agents are organic substances, either applied or produced by plants or microorganisms. The most important substances having this nature are hydroxamate siderophores, organic acids, and amino acids.
We mainly use amino acids as chelating agents in our products due to the numerous benefits they provide to plants and animals. Natural to plant and animal health, amino acids are more recognizable and easier to absorb. Essential amino acids work together aiding structure function and enzymatic function as well as improving reproduction. Amino acids increase health, lead to vigorous growth, and produce greater yields.
Our chelation process bonds nutrients with amino acids or other natural chelating agents in order to provide the best nutrition products possible. This process provides many added benefits such as increased nutrient mobility, nutrient precipitation prevention, and the avoidance of nutrient leaching.
Nutrient Mobility
Nutrients are necessary in plant and animal nutrition to increase health and growth. Nutrients can become difficult to absorb as they can become fixed or stuck outside of the plant and intestine. This is due to charge incompatibility between a plant and animal’s negative charge, and a nutrient’s positive charge. The chelate creates a compatibility between the two charges by encapsulating the positively charged nutrient and neutralizing it. The nutrient is then able to move freely into the plant or body. Chelation allows nutrients to be absorbed by plants or animals with ease.
Preventing Nutrient Precipitation
Nutrient loss occurs from precipitation caused by the interactions of nutrients. In the process of precipitation, an entirely available nutrient combines with another ion and forms an insoluble precipitate. To prevent this, the chelate encapsulates the nutrient, protecting it from coming into contact with the ion, keeping the nutrient available for absorption and slowly releases them when the plant or animal are ready for uptake.
Visually, we see precipitation happen when a grower mixes a product and the liquid is cloudy. As any grower will know, the mixture should be as clear as possible. The cloudier the mixture, the more precipitation is occurring along with a loss of nutrients. With chelation we prevent precipitation from happening and ensure the liquid remains clear and the nutrient remains ready for absorption.
Nutrient Leaching
In addition to all chelation does for plant and animal nutrition, chelation greatly helps the environment by combating nutrient leeching.
Nutrient leaching is problematic to plants and the environment for a multitude of reasons the main being ground water contamination. As water makes its way through soil it carries with it plant nutrients and pesticides. Though beneficial to plant life, as these nutrients make their way into water supplies, they create damage for the animals and people around it.
Chelation causes better absorption of nutrients into plants and animal intestines preventing a loss to runoff or nutrient excretion. Instead the products are more effective and have lower application rates allowing plants, the environment, and grower’s bottom line to thrive.
Where to Find Chelated Products
For more on chelation and the products that utilize chelation visit our product pages for plant nutrition products Biomin, Buffermate, Fulmag, and Nutrimin. As well as our chelated animal nutrition pages for Buffermin Glycinate, Buffermin Methionate, and Buffermin Proteinate.